Background
The Control-IQ Observational (CLIO) Study is currently an ongoing IRB-approved, single-arm, longitudinal study evaluating real-world use of Tandem’s t:slim X2 insulin pump with Control-IQ technology in people with T1D (PWT1D). Previous publications from CLIO have shown glycemic improvements in ethnically diverse groups of PWT1D after 3 months of using Control-IQ technology.
Method
We evaluated relationships between glycemic metrics, participants’ age, and previous insulin delivery method at 9 months after CLIO study start. Participants (N=1913) who had uploaded at least 21 days of Control-IQ feature usage data to Tandem’s t:connect® web application (US only) and had ≥75% CGM use were included in the analysis. Impact of baseline factors on sensor glycemic outcomes were analyzed. Differences between baseline HbA1c and GMI were compared using a Wilcoxon test.
Results
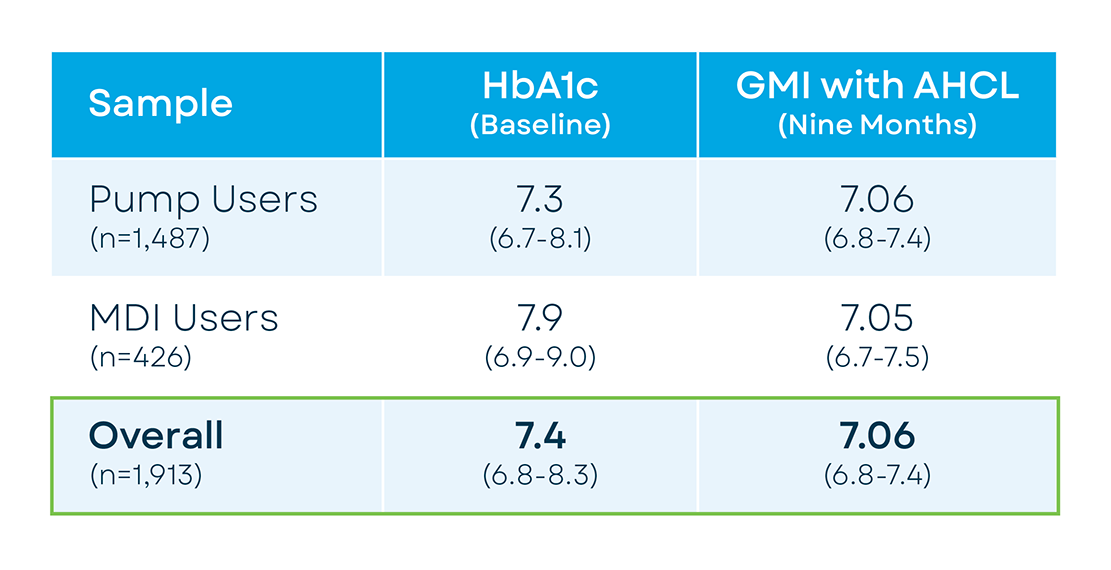
Baseline HbA1c (self-reported) for the overall sample was 7.4% (median, IQR=6.8-8.3). At post with Control-IQ technology, GMI reflected significant glycemic improvement (7.06%, IQR=6.75-7.42). Previous MDI users demonstrated 71.23% TIR (61.29-81.16) and overnight TIR=75.26% (64.62-84.5) while previous pump users showed TIR=70.43% (61.27-78.67) and overnight TIR=76.39(65.46-85.12). Participants aged ≥65 years, transitioning from prior pump therapy showed the lowest GMI (median=6.87, IQR=6.62-7.17) and the highest sensor time in range (TIR) (77.94%, IQR=69.29-83.8). Younger participants although reporting higher HbA1c at baseline (6–13-year-old, 7.8% (IQR=7.1-8.6); 14–17-year-old, 8% (IQR=7.1-9)) also showed significant glycemic improvements after 9 months of using Control-IQ technology (GMI=7.4 and 7.35, respectively).
Conclusion
Long-term use of Control-IQ technology demonstrated improved glycemic metrics irrespective of participants’ age and previous insulin delivery method.
| Participants’ age group (years) | Previous insulin delivery Method | n | Baseline HbA1c | GMI using Control-IQ technology | Significance |
|---|---|---|---|---|---|
| 6-13 | Pump User | 84 | 7.9(7.28-8.6) | 7.55(7.15-7.97) | p<0.001 |
| MDI User | 45 | 7.8(6.8-8.9) | 7.25(7.0-7.63) | p=0.02 | |
| 14-17 | Pump User | 163 | 8.1(7.2-9.0) | 7.4(7.0-7.89) | p<0.001 |
| MDI User | 63 | 7.8(6.8-9.0) | 7.19(6.66-7.6) | p<0.001 | |
| 18-30 | Pump User | 337 | 7.4(6.8-8.2) | 7.13(6.88-7.46) | p<0.001 |
| MDI User | 123 | 7.8(6.65-8.85) | 6.99(6.68-7.4) | p<0.001 | |
| 31-45 | Pump User | 415 | 7.2(6.55-8.0) | 7.01(6.71-7.33) | p<0.001 |
| MDI User | 100 | 8.25(7.0-9.65) | 6.97(6.74-7.44) | p<0.001 | |
| 46-64 | Pump User | 364 | 7.3(6.8-8.0) | 6.95(6.69-7.23) | p<0.001 |
| MDI User | 80 | 7.9(7.1-9.0) | 6.98(6.71-7.31) | p<0.001 | |
| ≥65 | Pump User | 124 | 7.0(6.6-7.52) | 6.87(6.62-7.17) | p<0.001 |
| MDI User | 15 | 7.5(6.85-7.95) | 7.13(6.88-7.24) | p=0.02 |
Median Glycemic Metrics
Product Documentation
Consult user guides for our pumps, infusion sets, predictive technology, and reporting app.
View DocumentationProduct Training
Use our education tutorials to train your patients or CDEs on our easy-to-use products.
View ResourcesPrescribing
Prescribing is easy with our customized referral process and insurance benefits check.
Learn MoreReferences
1. Breton MD, Kovatchev BP. One Year Real-World Use of the
Control-IQ Advanced Hybrid Closed-Loop Technology. Diabetes Technol Ther. 2021;23(9):601-608.
2.
Barnard K, et al. Patient-Reported Outcomes and Continuous Glucose Monitoring: Can we do better with
artificial pancreas devices? Diab Care. 2015;38(5):e70.
3. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop
control in type 1 diabetes. N Eng J Med. 2019;381(18):1701-1717.
4. Forlenza GP, Breton MD, Kovatchev
BP. Candidate selection for Hybrid Closed Loop Systems. Diabetes Technol Ther. 2021;23(11):760-762.
5. Habif S, Singh H, et al. 217-OR: Glycemic Outcomes by Ethnicity in Adults with Type 1 Diabetes Using
Control-IQ Technology: Early Results from the CLIO Study. Diabetes. 2021;70(Supplement_1):217-OR.
Important Safety Information
RX ONLY. The t:slim X2 pump and Control-IQ technology are intended for single patient use. The t:slim X2 pump and Control-IQ technology are indicated for use with NovoLog or Humalog U-100 insulin.
t:slim X2 insulin pump : The t:slim X2 insulin pump with interoperable technology is an alternate controller enabled (ACE) pump that is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The t:slim X2 pump is indicated for use in individuals six years of age and greater. Control-IQ technology: Control-IQ technology is intended for use with a compatible integrated continuous glucose monitor (iCGM, sold separately) and ACE pump to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons six years of age and greater.
| WARNING: Control-IQ technology should not be used by anyone under the age of six years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds. |
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of the t:slim X2 pump and Control-IQ technology must: use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The t:slim X2 pump, transmitter, and sensor must be removed before MRI, CT, or diathermy treatment. Visit www.tandemdiabetes.com/safetyinfo for additional important safety information.