In a clinical study, Control-IQ technology significantly improved time in range.
In the National Institutes of Health-funded Diabetes Closed Loop Trial, Protocol 3, time in range* for participants using a t:slim X2™ insulin pump with Control-IQ™ technology for six months averaged 71% per day compared to 59% for sensor-augmented pump therapy alone.1
Time in range* per day (24 hours) for study participants using Control-IQ technology on the t:slim X2 pump.1
Overnight time in range (12 a.m.-6 a.m.)* for study participants using Control-IQ technology.1
For patients who aren’t hitting HbA1c targets, the t:slim X2 pump with Control-IQ technology can help.
A patient’s struggle with glucose control impacts loved ones, caregivers, and even coworkers. Our easy-to-use t:slim X2 insulin pump with Control-IQ technology helps patients achieve targets, and better glucose management means happier patients. Regardless of baseline A1c, most study participants had a reduction over the 6‑month pivotal trial.1
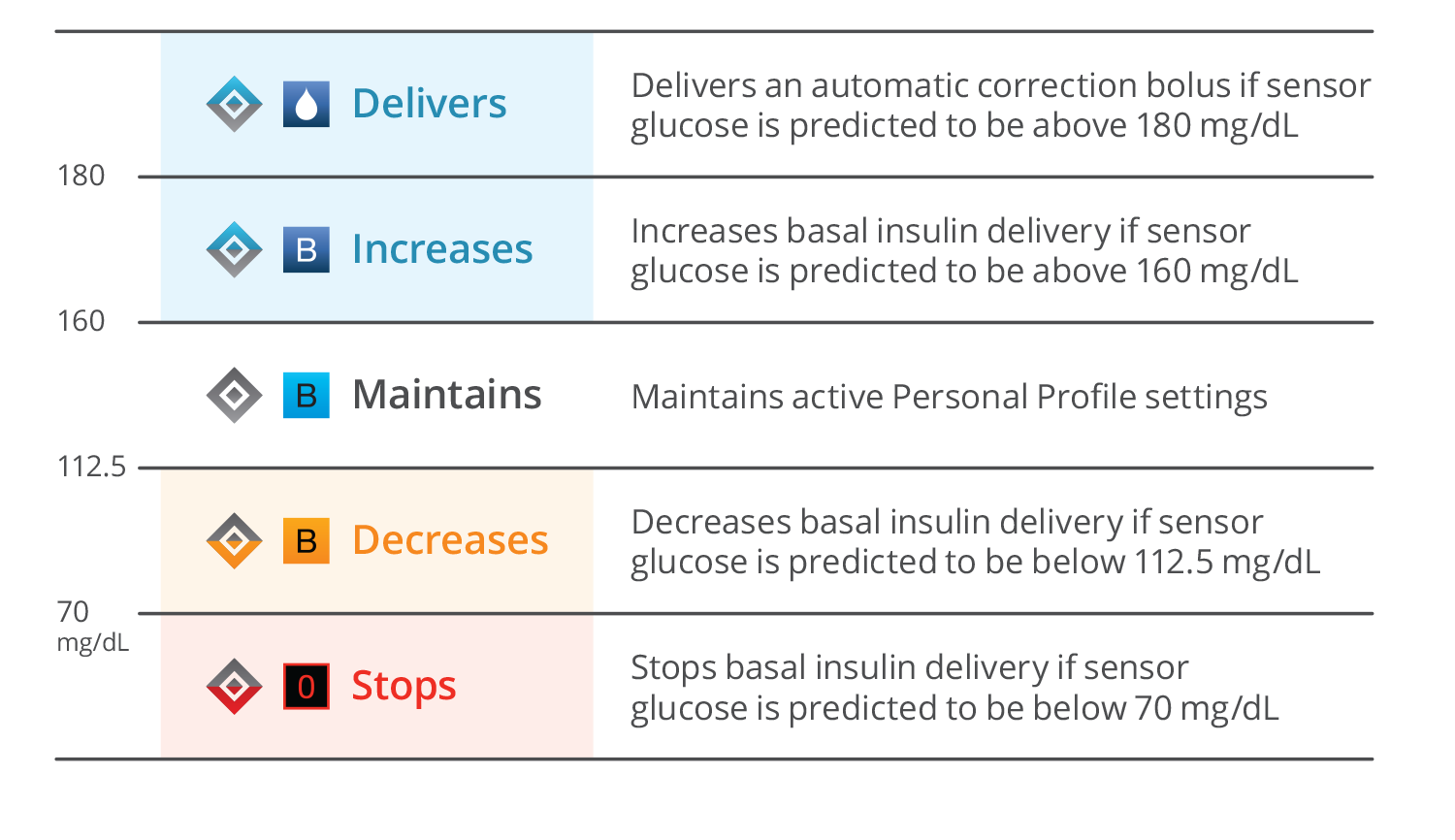
How does Control-IQ technology work?
Control-IQ technology helps increase time in range* using Dexcom G6 continuous glucose monitoring (CGM)† values to predict glucose levels 30 minutes ahead and adjust insulin delivery accordingly, including delivery of automatic correction boluses as needed.‡
Smooth Basal Rate Attenuation
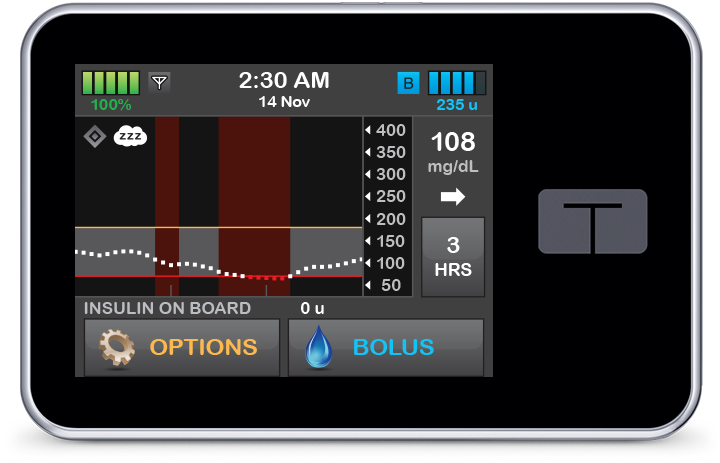
Control-IQ technology smoothly decreases or discontinues basal delivery based on falling CGM values to help prevent hypoglycemia.
Automatic Correction Boluses
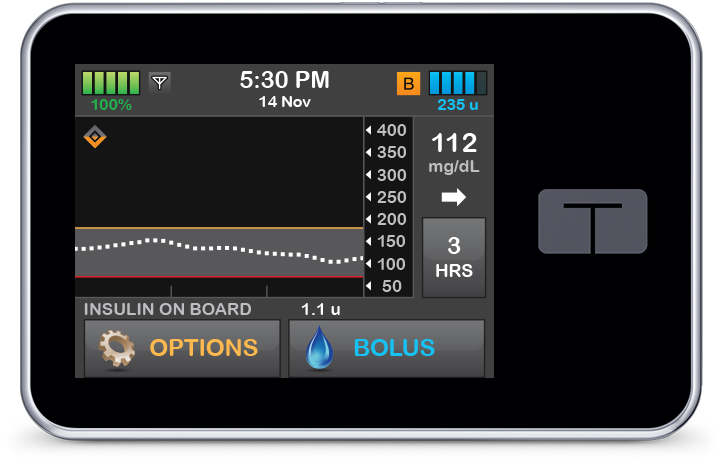
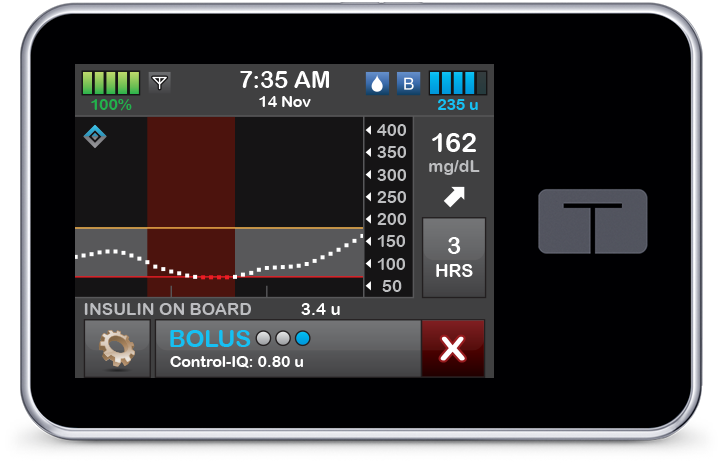
Control-IQ technology gradually increases basal insulin with increasing CGM values and administers 60% correction boluses once an hour as needed to help prevent hyperglycemia.
Sleep and Exercise Activity Settings
Control-IQ technology includes optional settings for Sleep and Exercise Activities that adjust treatment ranges to better match physiologic needs when active or sleeping.
Control-IQ technology offers a variety of features designed to help make control easier for your patients.
Automatic Basal Insulin Adjustments
Control-IQ technology automatically adjusts insulin levels based on Dexcom G6 CGM readings. The system uses a predictive algorithm to minimize wide fluctuations in glucose levels, increasing time in range.* In studies, the system was available 92% of the time.1
Automatic Correction Boluses
Control-IQ technology can deliver automatic correction boluses, a first for automated insulin delivery devices.
High Usability Ratings From Patients
On a 5-point scale, with 5 representing the optimal score, study participants rated the Control-IQ system a 4.8 for desire to continue use of the system, 4.7 for ease of use, and 4.5 for trust.2
Improved HbA1c and Mean Glucose
For patients struggling with glucose control, using Control-IQ technology may be able to help more easily achieve their targets. Study participants using Control-IQ technology saw statistically significant improvements in HbA1c and mean glucose values.1
Decreased Hyperglycemia
Time spent with glucose values above 180 mg/dL was only 27% in those using the t:slim X2 insulin pump with Control-IQ technology compared to 39% in the control group.1
Zero Fingersticks
When the t:slim X2 pump is integrated with Dexcom G6 CGM, zero fingersticks are required for calibration or mealtime dosing.§
§ Zero fingersticks required when using the t:slim X2 pump with Dexcom G6 CGM integration. If glucose alerts and CGM readings do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
* As measured by CGM.
† CGM sold separately.
‡ If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus with a target of 110 mg/dL and delivers 60% of that value. It will do this up to once per hour as needed.
§ Zero fingersticks required when using the t:slim X2 pump with Dexcom G6 CGM integration. If glucose alerts and CGM readings do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
References:
1. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med. 2019;381(18):1707-17. DOI: 10.1056/NEJMoa1907863
2. Brown S, et al. Clinical Acceptance of the Artificial Pancreas: The iDCL Trial. Poster presentation. 79th Scientific Sessions, American Diabetes Association, San Francisco, CA; June 9, 2019.
Important Safety Information
RX ONLY. The t:slim X2 pump and Control-IQ technology are intended for single patient use. The t:slim X2 pump and Control-IQ technology are indicated for use with NovoLog or Humalog U-100 insulin.
t:slim X2 insulin pump : The t:slim X2 insulin pump with interoperable technology is an alternate controller enabled (ACE) pump that is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The t:slim X2 pump is indicated for use in individuals six years of age and greater. Control-IQ technology: Control-IQ technology is intended for use with a compatible integrated continuous glucose monitor (iCGM, sold separately) and ACE pump to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons six years of age and greater.
| WARNING: Control-IQ technology should not be used by anyone under the age of six years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds. |
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of the t:slim X2 pump and Control-IQ technology must: use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The t:slim X2 pump, transmitter, and sensor must be removed before MRI, CT, or diathermy treatment. Visit www.tandemdiabetes.com/safetyinfo for additional important safety information.