Objective
To compare real-world glycemic outcomes between individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D) from a sample of early adopters of the t:slim X2™ insulin pump with Control-IQ® technology.
Method
The retrospective study included both T1D and T2D individuals who had recently updated their insulin pump software to initiate use of Control-IQ technology. Analysis included at least 14 days of pre- and 14 days of post-Control-IQ technology usage data that participants had uploaded to the t:connect® web application as of March 11, 2020.
Results
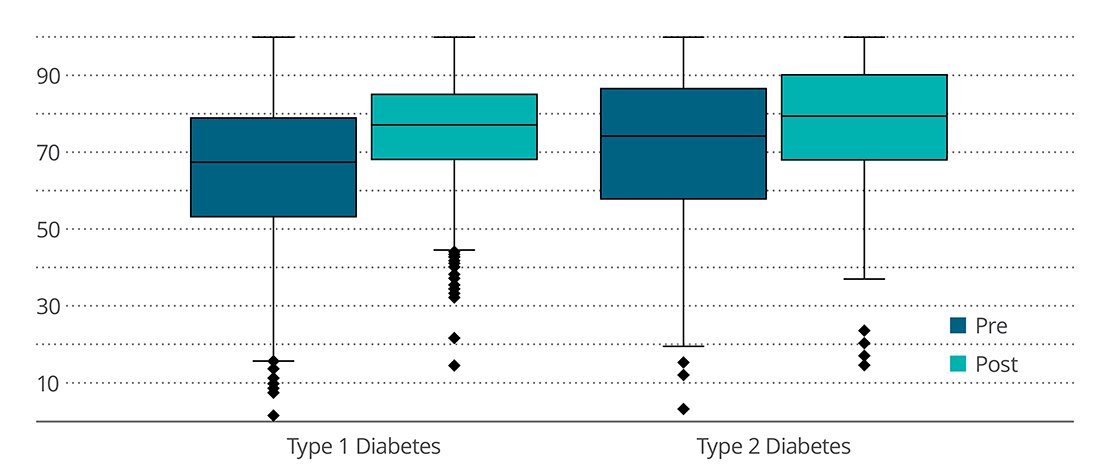
In the T1D subgroup (n=2,896), participants showed an increased sensor time in range, reduced time in sensor glucose <70 mg/dL, and reduced time in sensor glucose >180 mg/dL. In T2D subgroup (n=144), participants showed significant increase in sensor time in range and reduced time in sensor glucose >180 mg/dL. There was no change in time in sensor glucose <70 mg/dL. During 14 days of using Control-IQ technology, both T1D and T2D participants recorded 96% time in closed-loop automation.
Conclusion
These early results from the use of Control-IQ technology showed valuable improvements in time in range (based on sensor glucose values) and other glycemic variables for people with T1D and T2D. Improved sensor time in range, if maintained long-term, can help reduce the risk of diabetes-related complications.
Percentage of Time Sensor Spent in Range
Product Documentation
Consult user guides for our pumps, infusion sets, predictive technology, and reporting app.
View DocumentationProduct Training
Use our education tutorials to train your patients or CDEs on our easy-to-use products.
View ResourcesPrescribing
Prescribing is easy with our customized referral process and insurance benefits check.
Learn More* Tandem Diabetes Care † UC San Diego Design Lab Center for Health
References:
1. Reznik Y, et al. Insulin Pump for Type 2 Diabetes: Use and misuse of continuous
subcutaneous insulin infusion in type 2 diabetes. Diabetes Care. 2013; 36(Supp 2): S219-S225.
2. Singh P, et al. Insulin Pump in Difficult to Control Type 2 Diabetes: A Single Center, Five Years’
Experience. Cureus. 2018;10(8):e3240.
3. Brown SA, et al. Six-month randomized, multicenter
trial of closed-loop control in type 1 diabetes. N Eng J Med. 2019;381(18):1701-1717.
4. Beck RW,
et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care.
2018; 42(3): 400-405.
Important Safety Information
RX ONLY. The t:slim X2 pump and Control-IQ technology are intended for single patient use. The t:slim X2 pump and Control-IQ technology are indicated for use with NovoLog or Humalog U-100 insulin.
t:slim X2 insulin pump : The t:slim X2 insulin pump with interoperable technology is an alternate controller enabled (ACE) pump that is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The t:slim X2 pump is indicated for use in individuals six years of age and greater. Control-IQ technology: Control-IQ technology is intended for use with a compatible integrated continuous glucose monitor (iCGM, sold separately) and ACE pump to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. Control-IQ technology is intended for the management of Type 1 diabetes mellitus in persons six years of age and greater.
| WARNING: Control-IQ technology should not be used by anyone under the age of six years old. It should also not be used in patients who require less than 10 units of insulin per day or who weigh less than 55 pounds. |
Control-IQ technology is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Do not use Control-IQ technology if using hydroxyurea. Users of the t:slim X2 pump and Control-IQ technology must: use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts, alarms, and reminders. The t:slim X2 pump, transmitter, and sensor must be removed before MRI, CT, or diathermy treatment. Visit www.tandemdiabetes.com/safetyinfo for additional important safety information.