Objective
To better understand the formation of trust in automated insulin delivery (AID) systems by examining the relationship between trust and psychosocial outcomes and AID system usability.
Method
Individuals with diabetes responded to an online survey administered by dQ&A, which assessed variables of interest, trust, AID system perceived usability, psychosocial outcomes, basic demographics, and information on method of diabetes management.
Results
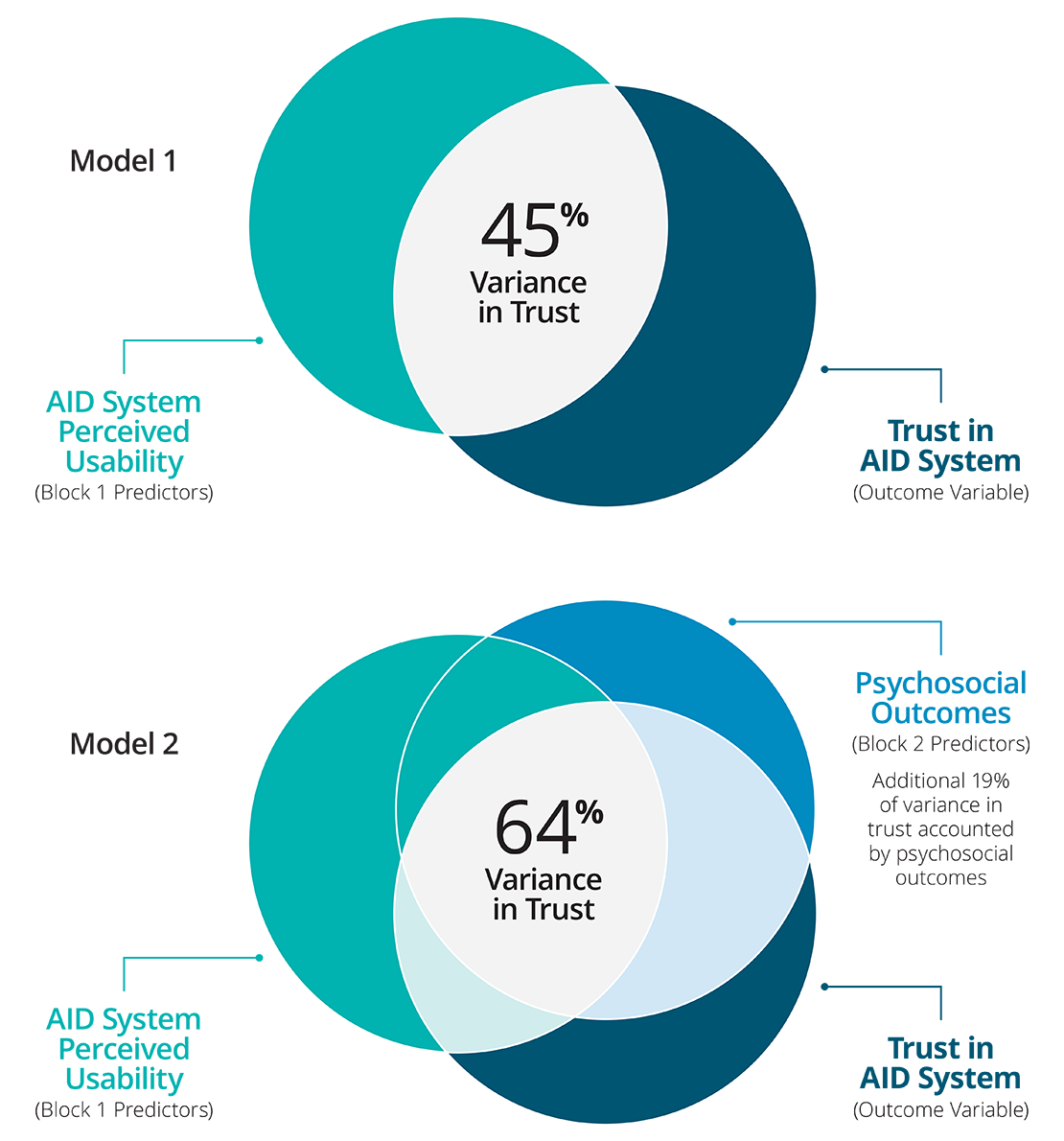
Hierarchical multiple regression analysis was used to ascertain the significance of ease of use (device level) and psychosocial (individual level) predictors of trust in AID systems. There were no issues with multicollinearity (all VIFs < 3). Ease of use accounted for 45% of variance in trust, F (3,382) = 106.74, p < .001. After psychosocial predictors were added variance accounted for was 64%, explaining an additional 19% of unique variance in trust, F (3,379) = 65.46, p < .001. Specifically the predictor variables (i.e., easy to use, too complicated, satisfaction, helps me have good BG control, and helps me sleep better at night) all significantly predict trust in AID systems.
Conclusion
Study findings suggest that both AID system perceived usability and psychosocial outcomes are distinctly informative and instrumental in the development of trust in AID systems.
Hierachical Regression Results
Product Documentation
Consult user guides for our pumps, infusion sets, predictive technology, and reporting app.
View DocumentationProduct Training
Use our education tutorials to train your patients or CDEs on our easy-to-use products.
View ResourcesPrescribing
Prescribing is easy with our customized referral process and insurance benefits check.
Learn More* Tandem Diabetes Care, Inc. † dQ&A
Important Safety Information
Caution: Federal (USA) law restricts the t:slim X2 insulin pump and the t:slim X2 insulin pump with Basal-IQ technology to sale by or on the order of a physician. The t:slim X2 insulin pump with Basal-IQ technology (the System) consists of the t:slim X2 insulin pump, which contains Basal-IQ technology, and a compatible integrated continuous glucose monitor (iCGM, sold separately). The t:slim X2 insulin pump is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The t:slim X2 insulin pump can be used solely for continuous insulin delivery and as part of the System. When used with a compatible CGM, the t:slim X2 insulin pump with Basal-IQ technology can be used to suspend insulin delivery based on CGM sensor readings. The pump and the System are indicated for use in individuals 6 years of age and greater. The pump and the System are intended for single patient use. The pump and the System are indicated for use with NovoLog or Humalog U-100 insulin. The System is not indicated for use in pregnant women, people on dialysis, or critically ill patients. Users of the pump and the System must: be willing and able to use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts. The t:slim X2 pump, transmitter, and sensor must be removed before MRI, CT, or diathermy treatment. For additional important safety information, visit www.tandemdiabetes.com/safetyinfo.