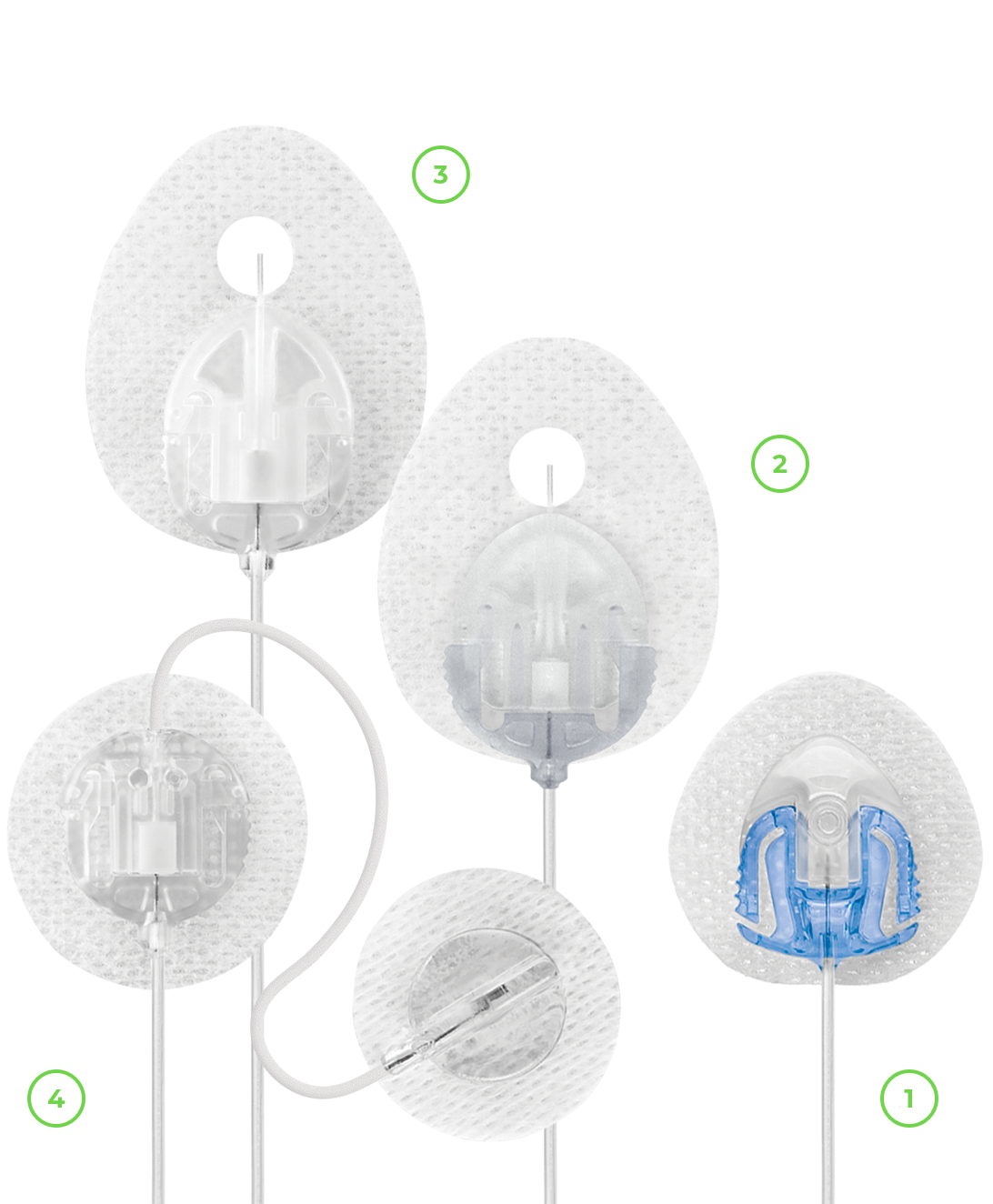
* Dexcom CGM sold separately.
† 38% smaller than MiniMed 770G. Data on file, Tandem Diabetes Care.
‡ Zero fingersticks required when using the t:slim X2 pump with Dexcom G6 CGM integration. If glucose alerts and CGM readings do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
§ Additional feature updates are not currently available for the t:slim X2 insulin pump with Control-IQ technology. Control-IQ technology is available only to customers who reside in the United States or Canada, and other select geographies. Future updates for all or some of Tandem’s products may not be developed and may not be offered everywhere and would be subject to applicable regulatory approvals. Software updates are only available to customers who are in warranty at the time they update their pump. Additional training may be required to access certain future software updates. Charges may apply. Tandem may discontinue select software and features over time at its discretion.
Important Safety Information
The pump and each System below is indicated for use in individuals six years of age and greater. The pump and each System is intended for single user use. The pump and each System is indicated for use with U-100 insulin only.
The t:slim X2 insulin pump with Basal-IQ technology (the Basal-IQ System) consists of the t:slim X2 insulin pump, which contains Basal-IQ technology, and a compatible continuous glucose monitor (CGM, sold separately). The t:slim X2 insulin pump is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The t:slim X2 insulin pump can be used solely for continuous insulin delivery and as part of the Basal-IQ System. When used with a compatible CGM, the t:slim X2 insulin pump with Basal-IQ technology can be used to suspend insulin delivery based on CGM sensor readings.
The t:slim X2 insulin pump with Control-IQ technology (the Control-IQ System) consists of the t:slim X2 insulin pump, which contains Control-IQ technology, and a compatible continuous glucose monitor (CGM, sold separately). The t:slim X2 insulin pump is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The t:slim X2 insulin pump can be used solely for continuous insulin delivery and as part of the Control-IQ System. When used with a compatible CGM, the t:slim X2 insulin pump with Control-IQ technology can be used to automatically increase, decrease, and suspend delivery of basal insulin based on CGM sensor readings and predicted glucose values. The t:slim X2 insulin pump with Control-IQ technology can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold.
Warnings
Warning: Control-IQ technology should not be used by anyone under the age of six years old. It should also not be used in users who require less than 10 units of insulin per day or who weigh less than 25 kilograms.
Do not use the t:slim X2 insulin pump with Control-IQ technology if using hydroxyurea. Neither System is indicated for use in pregnant women, people on dialysis, or critically ill users. Users of the pump and either System must: be willing and able to use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts. The t:slim X2 pump and the CGM transmitter and sensor must be removed before MRI, CT, or diathermy treatment. Visit www.tandemdiabetes.com/safetyinfo for additional important safety
information.