Objective
To examine sleep related implications of new-age diabetes management devices including Basal-IQ technology in people with T1D and T2D.
Method
We analyzed perceived quality of sleep and device-related satisfaction in participants from a diabetes research company’s (dQ&A) 2019 US-based panel.
Results
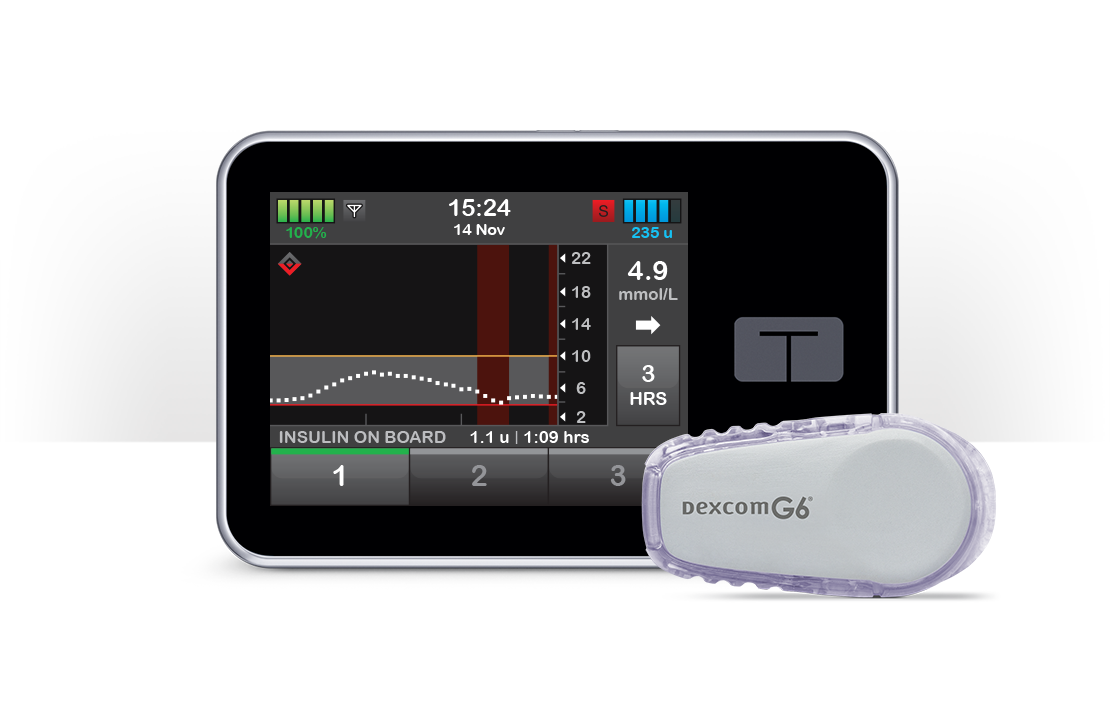
Participants using the t:slim X2™ insulin pump with Basal-IQ® technology reported significantly better perceived quality of sleep and satisfaction with their insulin delivery device compared to participants using other insulin delivery devices.
Conclusion
Perceived quality of sleep was an important determinant of device-related satisfaction in this sample.
Product Documentation
Consult user guides for our pumps, infusion sets, predictive technology, and reporting app.
View DocumentationProduct Training
Use our education tutorials to train your patients or CDEs on our easy-to-use products.
View ResourcesRecommending
Recommending the t:slim X2 insulin pump is easy with our customized referral process and insurance benefits check.
Learn More* Tandem Diabetes Care, San Diego. †dQ&A - The Diabetes Research Company, San Francisco. ‡ Questionnaire item “My insulin delivery device helps me sleep better at night” was scored on a Likert response scale (1-10) with higher values suggesting better perceived quality of sleep.
References
1. Arora T, Taheri S. Sleep Optimization and Diabetes Control: A Review of the Literature.
Diabetes Ther 2015;6(4):425-468.
2. Lee SWH, et al. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis.
Sleep Med Rev 2017;31:91-101.
3. Schnurbein JV, et al. Sleep and glycemic control in adolescents
with type 1 diabetes. Pediatr Diabetes 2018;19(1):143-149.
4. Monzon A, et al. Sleep and type 1 diabetes in children and adolescents: Proposed theoretical model and clinical implications.Pediatr Diabetes 2019;20(1):78-85.
5. American Diabetes Association: Standards of medical care in diabetes. Diabetes Care 2017;40:S1–S135.
6. Perez KM, et al. Sleep in Type 1 Diabetes:Implications for Glycemic Control and Diabetes Management. Curr Diab Rep. 2018;18(2):5.
Important Safety Information
The t:slim X2 insulin pump with Basal-IQ technology (the System) consists of the t:slim X2 insulin pump, which contains Basal-IQ technology, and a compatible continuous glucose monitor (CGM, sold separately). The t:slim X2 insulin pump is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The t:slim X2 insulin pump can be used solely for continuous insulin delivery and as part of the System. When used with a compatible CGM, the System can be used to suspend insulin delivery based on CGM sensor readings. The pump and the System are indicated for use in individuals six years of age and greater. The pump and the System are intended for single user use. The pump and the System are indicated for use with NovoRapid, Admelog, or Humalog U-100 insulin. The System is not indicated for use in pregnant women, people on dialysis, or critically ill users. Users of the pump and the System must: be willing and able to use the insulin pump, CGM, and all other system components in accordance with their respective instructions for use; test blood glucose levels as recommended by their healthcare provider; demonstrate adequate carb-counting skills; maintain sufficient diabetes self-care skills; see healthcare provider(s) regularly; and have adequate vision and/or hearing to recognize all functions of the pump, including alerts. The t:slim X2 pump and the CGM transmitter and sensor must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes.com/safetyinfo for additional important safety information.